- H. Faulkner e,S J3ckson, G. Fatania, and C. A. Leicht
Nationa Centre for Eports and Exercise Medicine, S:hool of Eport Exercise and 1-Eath 8:ienoos, Loughborough University, Loughborough, Leice:tersiire, Uiited Kingdom
ABSTRACT
Increasing physical activity remains the most widely publicized way of improving health and wellbeing. However, in populations that benefit most from exercise (EX), adherence is often poor and alternatives to EX are important to bring about health improvements. R3cent work suggests a role for passive heating (PH) and heat shock proteins (HEP) in improving cardio-metabolic health. The aim of this study was to investigate the expression of HEP?0 and interleukin-6 in response to either EX or PH and the subsequent effect on glucose control. Fourteen males volunteered and
were categorized lean (BMI 23.5 § 2.2 kg¢n1 2) o”r overweight (29.2 § 2.7 kg¢n1 2) and completed
60 minutes of either moderate cyding at a fiXed rate of metabolic heat production (EX) or warm water immersion in 4 water (PH). Extracellular HEP?0 increased from baseline in both conditions with no differences between PH (0.98 § 1.1 ng¢nL’ 1) or EX (0.84 § 1.0 ng¢nL’ 1, p D 0.814). IL-6 increased following both conditions with a two-fold increase after PH and four-fold after EX. Ehergy expenditure increased by 61.0 § 14.4 kcal¢11 1 (79%) after PH. Peak glucose concentration after a
meal immediately following PH was reduced when compared with EX (6.3 § 1.4 mmol¢..1 1 versus
6.8 § 1.2 mmol¢..1 1; p < 0.05). There was no difference in 24-hour glucose area under the curve (AUG) between conditions. These data indicate the potential for thermal therapy as an alternative
treatment and management strategy for those at risk of developing metabolic disease where adherence, or ability to EX, may be compromised.
Introduction
Overweight and obesity are characterized by chronic inflammation and impairments to insulin sensitivity and glucose control. A multitude of factors contribute to enhancing health and wellbeing; increasing physical activity remains the most widely publicized way of doing so. However, in populations that benefit most from exercise (EX), adherence is often poor, most likely due to medical conditions and disability,1 poor motivation and a lack of convenience2. There are many factors involved in the development and treatment of insulin resistance, with the roles played by both thermal therapy and heat shock proteins (HSPs) receiving increased attention.4 7 Furthermore, it has been suggested that HSPs may provide an early indicator of the onset of metabolic diseases, such as type 2 diabetes (T2DJvI),8 9 while also acting as a possible bio marker for the chronicity of diabetes.10
HSPs are synthesized in response to a number of physiological stressors,l H 3 and are individually characterized by their molecular weight. The most widely studied HSP in humans is the 70 kDa family of HSPs which includes both the constitutive HSP73 and inducible HSP72 forms. HSPs have wide-ranging functions including their roles as thermo-protectants and volvement in protein synthesis.14 The resting level of extracellular HSP70 (eHSP70) appears to be related to adiposity” and inflammation with elevations reported in obesity and T2DM, whereas resting intra cellular HSP70 (iHSP70) is decreased in T2DM.9 A reduction in iHSP70 is an important component in the vicious cycle of chronic inflammation and reduced resistance.17 18 Indeed, a reduction in iHSP70 has been insulin signalling15 16 and the development of insulin reported in T2DM and correlates to insulin resistance and glucose disposal rates.19 2
eHSP70 is increased following acute EX13 with the magnitude of change dependent on both the duration and intensity.21,22 A core temperature (Tc) threshold for elevations in eHSP70 has been suggested, with a 0.8-1.5 increase in Tc being required in order to elevate eH SP70.,23 24 Recently, thermal therapy has been demonstrated to stimulate HSP70 production, although to a lesser extent than following EX,4’24 and may offer an alternative way by which the positive effects ofHSP may be induced.9 The influence of thermal therapy on glycemic regulation and metabolic disease has primarily been inves tigated using rodent models.17’18’25 7. However, there are human data to suggest that local heating at the site of insulin infusion improves postprandial insulin action28·29 and may benefit long-term glycemic con trol29·30 and cardiovascular health in hum ans.31 However, the mechanisms by which such an effect may occur in humans remain to be elucidated. Chung et al. were the first to demonstrate that passive heating (PH) of mice resulted in an elevation in iHSP70 which was protective against the deleterious effects of con suming a high-fat diet.18 They report that in response to a high-fat diet there was an increase in the phos phorylation of c-Jun N-terminal kinase (JNK). Such an increase has been linked to impaired insulin sensi tivity due to JNK binding with the insulin receptor substrate, thus preventing the initiation of the insulin signaling cascade and resulting in an elevation in plasma glucose (For review, the reader is directed to ref [32]). Importantly, Chung et al. demonstrated that PH attenuated hyperinsulinemia, glucose intolerance and insulin resistance and as a direct consequence of elevations in HSP70 and was closely associated with prevention of JNK. phosphorylation. Therefore, there is a need to investigate how well data from these rodent models translate into human participants, with a specific focus on the ability of thermal therapy to ele vate HSP70 and improve glycemic control.
HSPs may impact on the inflammatory state by directly inducing pro-inflammatory cytokines. Regular EX has been demonstrated to have a positive effect on the inflammatory profile that is suggested to occur asa consequence of the repeated exposure to an anti inflammatory environment following each EX bout.33This may occur as a consequence of secretion of pro inflammatory cytokines, including interleukin-6 (IL-6) that may in turn up-regulate anti-inflammatory cytokines.34 Importantly, it has been shown that HSP70 independently induces pro-inflammatory cytokines.35·36 Therefore, thermal therapy may be able to replicate some of the anti-inflammatory benefits of EX, which could help reduce the level of inflammation evident in many types of chronic disease. While EX has significant benefits to improving health, individuals with many types of chronic disease often experience low EX tolerance and poor rates of adherence.1·37 Based on the above animal literature, there is evidence to suggest that thermal therapy may replicate some of the health benefits of EX and allevi ate some of the comorbidities often associated with chronic diseases such as T2DM.38 If successful, the implementation of thermal therapy may help to lessen some of the financial burden of treating chronic by potentially reducing the dependence on pharmacological interventions. Thermal therapy could offer a simple home-based intervention that may appeal to individuals unable or unwilling to participate in regular EX
The primary aim of this study was to investigate the expression of eHSP70 in response to either EX or thermal therapy in the form of PH via warm water immersion. A secondary aim was to investi gate the effect of body composition on the eHSP70 response. Finally, we wished to investigate the potential effect of PH on glycemic control in response to replicated dietary intake. It was hypothesized that both EX and PH would elevate eHSP70, with a larger magnitude of change follow ing EX. It was further hypothesized that there would be a differential response in eHSP70 between lean and overweight participant groups.
Methods
Participants
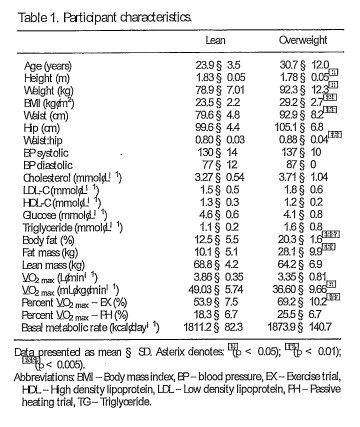
A total of 14 males volunteered to participate in the study and were split into two groups: (i) Lean (LEAN, n D 7; BMI < 25 kg¢n1 2, body fat < 15% and fat mass < 12 kg) and (ii) Overweight (OW, n D 7; BMI > 27.0 kg¢n 1 2, body fat > 20% and fat mass > 25 kg). Participant characteristics are provided in Table 1. All participants were healthy non-smokers with no history of cardiovascular, hematological or metabolic disorders and had been weight stable for [ID 3 months. Participants were habitually inactive; per forming less than 1.5 hours of structured physical activity per week and had avoided any hot weather
exposure in the previous two months, including fre quent sauna or spa use.
Ethical approval
Full ethical approval was granted by the Loughbor ough University Ethical Advisory Committee. All pro cedures conformed to the principles defined in the Declaration of Helsinki. Participants were fully informed of the experimental protocols and any potential risks were identified before they provided the:ir written consent to participate.
Experimental overview
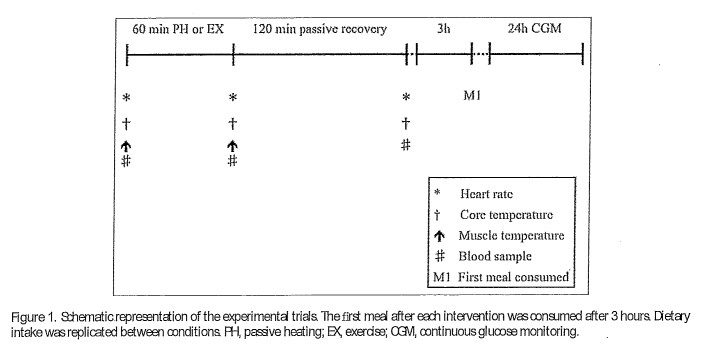
Participants visited the laboratory on three occasions. The preliminary visit to the laboratory consisted of initial measures including a blood profile, body com position and both submaximal and maximal oxygen uptake tests (Y.02 max test). Visits two and three com prised the experimental trials which consisted of either 60 minutes of PH via warm water immersion (PH), or 60 minutes of EX at a fixed rate of metabolic heat production (EX) which were completed in a counterbal anced order and were matched for DTc, On arrival, participants were asked to void the:ir bladder and were weighed in minimal clothing and then fully instru mented. The first blood sample was then collected and muscle temperature measured. Participants then com pleted either PH or EX as detailed below. On comple tion of each trial, a second blood sample and muscle temperature measure were obtained, with a final blood sample being taken 2 hours after completion (Figure 1). Experimental trials were separated by a minimum of 5 days to reduce any acclimation effect 39 and to minimize any effect of prior EX or heating upon insulin sensitivtiy.40 All trials were completed at the same time of day to minimize effects of c:ircadian variation.
Preliminary visit
Participants arrived in a fasted state and a capillary blood sample was drawn for assessment of total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (illL C), glucose and triglycerides (TG) (Cardiochek, Polymer Technology Systems, Indianapolis, IN). Participants’ height and weight were recorded (Seca 360, Birmingham, UK), and BM[ calculated. Body composition was determined with skinfold callipers (Harpenden, Warwickshire, UK) using the seven site formula. 41 Participants then completed a sub maximal EX test on an electronically braked cycle ergometer (Lode Excalibur Sport, Groningen, Neth erlands) with an online gas analyzer (Metamax 3B, Cortex Biophysik GmbH, Germany). The test compised four stages, increasing by· 20 W every 4 minutes. Y…02 and‘ \l .C0 2 were determined over a 30-second rolling average from the final minute of each stage. On completion of the submaximal test, participants were given a minimum of a 10-minute recovery period prior to commencing the YO2 max test. This test used a continual ramp protocol at a rate of 20 W¢nin1 1 until the participant reached volitional exhaustion. YO2 max was determined over a 30-second rolling average from the final minute of the test.
Experimental trials
Seventy-two hours prior to the start of each experi mental trial, participants reported to the laboratory where they were fitted with a continuous glucose monitor device (CGM; Freestyle Libre, Abbott Lab oratories, Berkshire). The sensor was inserted on the posterior aspect of the upper arm, with intersti tial glucose measured every 15 minutes. CGM data were analyzed for 2-hours post meal glucose AUC and peak glucose concentration.42 Participants were provided with scales (HoMedics Group Salter, Kent) and detailed instruction on how to measure dietary intake in order to accurately complete a 3- day diet diary during the first trial, which com menced 24 hours before and ended 24 hours after completion of trial 1. Participants were instructed to consume the first meal after each condition fol lowing a standardized 3-hour time period. Partici pants were required to replicate their dietary intake and timing of meals for the second trial.
Exercise trial
Participants performed 1-hour cycling at a fixed rate of metabolic heat production (1:1..proo) equivalent to 7 W/kg in an environmental chamber (25.6 § 0.7Ue; 49.8 § 3.8% relative humidity, rh). The initial work load was determined from the submaximal test and external workload was manipulated throughout the 60 minutes in order to maintain 1:1..proo at 7 W/kg. Par ticipants completed the EX at a self-selected cadence. Y 02 and YCO2 were recorded throughout using an online gas analyzer. Airflow was provided by three fans stacked vertically, positioned 2.5 min front of the ergometer at an air speed of 1.5 m¢;1 1 .
Passive heating trial
Participants were seated in a water bath (40.2 §0.2 ) and immersed up to the waist for 1 hour. Water was circulated throughout to ensure main tenance of water temperature. V.D2 and V….CO2 were recorded throughout. Ambient conditions during the trial were 23.9 § 0.9 Ue; 49.9 § 4.4% rh. Owing to the effect of temperature and duration on HSP70 and IL-6 expression, we attempted to match the change in core temperature between conditions by eliciting a 1 increase in core temperature. Further more, the duration of both EX and PH was matched to limit the effect of intervention exposure time on key outcome measures.
Instrumentation
Core temperature (Tc) was measured using a rectal thermistor (Grant Instruments Ltd, Cambridge) inserted 10 cm beyond the anal sphincter. Wireless skin sensors (iButtons, DS1922, California, USA) were applied and secured by Medipore tape (3M, Berkshire, UK.). Mean skin temperature (Ts<./ was calculated according to the formula of Ramanathan.43 Partici pants wore a heart rate monitor throughout (RS800,Kempele, Polar, Finland).
Muscle temperature
Muscle temperature (Tm) was measured in the right vas:us lateralis using a solid needle probe (MKA08050A275TS Ellab, Copenhagen, Denmark) immediately pre- and post-trial. Following standard sterile procedure, the needle probe was first inserted to an initial depth of 3 cm beyond the muscle fascia where the temperature was allowed to stabilize before the probe was withdrawn to 2 cm and then 1cm depths, with the temperature recorded at each depth.
Blood sampling
Venous blood samples were collected byvenepuncture from an antecubital vein in the right arm into a 10 mL Vacutainer that had been pre-cooled and pre-treated with K3EDTA (BD Biosciences, San Diego, USA). Samples were obtained before and on completion of each heating protocol with a final sample taken 120 minutes later (Figure 1). Samples were stored on ice until they were centrifuged at 3,500 rpm for 10 min at 4Ue and the plasma stored at i 80Lle until subsequent analysis.
Enzyme-linked immunosorbent assays Plasma HSP70 (ENZ-101, AMP’D6 hs HSP70, Enzo Life Sciences, Exeter, UK) and IL-6 (HS600B, Quantikine6 HS IL-6, R&D Systems, Abingdon, UK) concentrations were analyzed using enzyme-linked immunosorbent sandwich assays (EilSAs) according to the manufacturer’s instructions. Plasma HSP70 and IL-6 concentrations were determined in relation to a four-parameter standard curve (version 6.0 GraphPad Software, La Jolla, CA, USA). All samples were ana lyzed in duplicate with a mean intra plate coefficient of variation (CV) of 4.5% for HSP70 and 5.8% for IL-
The interplate CV was 6.3% for HSP70 and 6.1% for IL-6.
Calculations
Energy expenditure during PH and EX was calculated via indirect calorimetry. Heat balance parameters were estimated via partitional calorimetry and are presented as the mean value for each condition. All parameters were calculated in W/m 2 but presented as W/kg where appropriate. The rate of metabolic energy expenditure was estimated as where RER is the respiratory exchange ratio and Eb and Ej, represent the energy equivalent of carbohydrate (21.13 kJ) and fat (19.69 kJ) respectively per liter of 0 2 consumed per minute (L¢nin1 1 ) . tlpr00 was deter mined as the difference between M and the external work rate (W)
Mean body ternperature (DTb) was estimated using the three-compartment model as follows: D1\ D . 0 :63 D Tc/ C . 0 :24 DT 9/ C . 0 :13 D T ml (3) where DTc represents the change in core temperature, DTfl< the change in mean skin temperature and DTm the change in muscle temperature at a depth of3 cm.44 AUC was calculated using the trapezoid method. S:atistical analysis The normality and distribution of data was assessed using the Shapiro-Wilk normality test. Where data failed to meet the criteria of normal distribution, the data were log transformed. Mean participant charac teristics were compared using independent samples t tests. Tc, T fl<, Tm, HSP70, IL-6 were analyzed using repeated measures ANOVA. Where significance was obtained post hoc tests were completed using Sidak’s test for multiple comparisons. l:i..proct, DTb and energy expenditure were analyzed using one-way ANOVA with Sidak’s test for multiple comparisons. Correla tional analysis was done using Pearson’s correlation coefficient. Linear regression was used to analyze the relative contributions of DTc, DTsk, DTm and DTb to DHSP70 and DIL-6. The value was employed to determine the variance explained by each predictor variabie. Where reported, the adjusted value is pro vided for multiple linear regression in order to account for the number of predictor variables in the model. All statistical analyses were performed using GraphPad Prism (version 6.0 GraphPad Software, La Jolla, CA, USA). All data are presented as mean § SD unless otherwise stated. p values illb.05 were consid ered statistically significant. Effect sizes (ES) corrected for bias using Hedge’s g were calculated as the ratio of the mean difference to the pooled standard deviation of the difference, with 95% confidence intervals (95% CI) for differences also presented. The magnitude of the ES was classed as trivial (< 0.2), small (0.2-0.6), moderate (0.6-1.2), large (1.2-2.0) and very large crn:h.o).45
Results
l::iprod· There were differences in l::ipr00 between PH (1.9 § 0.2 W/kg) and EX (7.3 § 0.5 W/kg, p < 0.001). l::iprod was successfully matched between groups for both PH (LEAN 2.0 § 0.2 W/kg, OW D 1.8 § 0.2 W/ kg, p D 0.433, CI D i 0.03 to 0.43, ES D 0.9) and EX (LEAN 7.3 § 0.4, OW 7.2 § 0.5 W/kg; p D 0.763, CI D i 0.63 to 0.43, ES D 0.21). However, when lean body mass is accounted for, differences in l::iprod were evident (IEAN 8.4 § 0.4 W /k g/LBM1 1; OW 9.8 § 1.6 W/kg!IBM1 1, p < 0.05, CID 0.04 to 2.76, ES D 1.1).eHs:170. There was a main effect of time (p < 0.005) but not condition (p D 0.887) on the eHSP70 response (Figure 2A). The increase in eHSP70 follow ing PH and EX was 23% ·and 24%, respectively (Figure 2B). Across conditions DeHSP70 correlated with mean Tm (r D 0.485, p < 0.05). DeHSP70 was negatively correlated to body mass (r D i 0.400, p < 0.05). Of the thermal variables, DTm provided the best predictor of DHSP70, with the DTm explaining 24.7% of the total variance in DHSP70 (r D 0.498, p < 0.01) Body mass also predicted DHSP70, explaining 16.4% of the total variance (r D i 0.405, p < 0.05). When combined, DTm and body mass provided the strongest predictor, accounting for 28% of the variance in DHSP70 (Adjusted D 0.280, r D 0.585, p < 0.01). IL-6. There was a main effect of time (P < 0.001), condition (P < 0.05) and an interaction effect (P < 0.01, Figure 2C) on IL-6 concentration. IL-6 was higher immediately following EX (1.74 § 1.79 pg¢nL1 1 ) compared to PH (0.97 § 0.86 pg¢n L1 1, p <0.0001, ES D 0.5) and equated to an increase of346% after EX and 118% following PH (Figure 2D) from baseline values. IL-6 had returned to baseline at 2-hours post for PH (0.58 § 0.47 pg¢nL’ 1 and EX (0.73 § 0.79 pg¢n L1 1 ) . When considering differences between IEAN and OW, there was a main effect of time (p < 0.05) but not group (p D 0.533) on the IL-6 response. None of the thermal variables, nor body mass, provided an effective model for predicting the change in IL-6.
Energy expenditure
There were no differences in resting metabolic rate between IEAN (1811.2 § 82.3 kca1¢iay1 1 ) and OW (1873.9 § 140.7 kcal¢iay1 1; p D 0.330, ES D 0.5).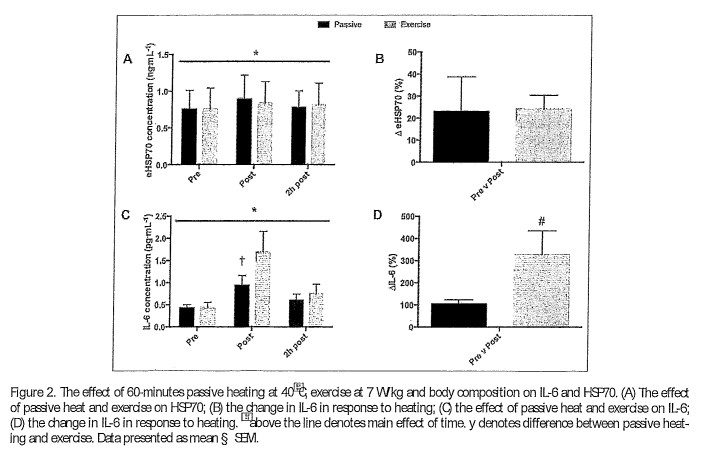 There was an overall effect of condition on energy expenditure during heating (p < 0.0001) and an inter action effect (p < 0.05). PH increased energy expenditure by 61.0 § 14.4 kcal¢i.1 1 compared to rest (p < 0.0001, ES D 4.8), which equates to a 79.5% increase. EX resulted in an additional energy expenditure of 556.3 § 92.0 kcal¢i.1 1 compared to rest (p < 0.0001, ES D 7.1), equating to a 721% increase. EX energy expenditure was greater for OW compared to IBAN (11.3 § 1.9 kcal¢nin1 1 versus 9.8 § 0.9 kcal¢nin1 1, respectively, p < 0.05, ES D 1.0), resulting in an additional caloric expenditure from rest of 597.7 §107.9 kcal for OW (748%), and 515 § 52.1 kcal for IBAN (681%). During PH, there was no difference in the increase in energy expenditure between IBAN and OW (2.3 § 0.3 kcal¢nin1 1 versus 2.3 § 0.3 kcal¢nin1 1, respectively, p D 0.997, ES D 0.0). Caloric expenditure increased from rest by 63.4 § 16.6 kcal (84%) and 58.5 § 12.7 kcal (74%) kcal for IBAN and OW, respectively although there was no difference between groups (p D 0.623, ES D 0.3).
There was an overall effect of condition on energy expenditure during heating (p < 0.0001) and an inter action effect (p < 0.05). PH increased energy expenditure by 61.0 § 14.4 kcal¢i.1 1 compared to rest (p < 0.0001, ES D 4.8), which equates to a 79.5% increase. EX resulted in an additional energy expenditure of 556.3 § 92.0 kcal¢i.1 1 compared to rest (p < 0.0001, ES D 7.1), equating to a 721% increase. EX energy expenditure was greater for OW compared to IBAN (11.3 § 1.9 kcal¢nin1 1 versus 9.8 § 0.9 kcal¢nin1 1, respectively, p < 0.05, ES D 1.0), resulting in an additional caloric expenditure from rest of 597.7 §107.9 kcal for OW (748%), and 515 § 52.1 kcal for IBAN (681%). During PH, there was no difference in the increase in energy expenditure between IBAN and OW (2.3 § 0.3 kcal¢nin1 1 versus 2.3 § 0.3 kcal¢nin1 1, respectively, p D 0.997, ES D 0.0). Caloric expenditure increased from rest by 63.4 § 16.6 kcal (84%) and 58.5 § 12.7 kcal (74%) kcal for IBAN and OW, respectively although there was no difference between groups (p D 0.623, ES D 0.3).
8Abstrate oxidation
Total EX carbohydrate oxidation was higher for OW (125.3 § 20.8 g) compared to IBAN (92.5 § 28.2 g; p < 0.05, ES D 1.3). There was a moderate effect of body mass on total carbohydrate oxidation in PH between OW (7.5 § 4.2 g) and IBAN (13.2 § 8.3 g, ES D 0.83) although this did not reach significance (p D 0.13). There was no difference between OW and IBAN in total fat oxidation during EX (p D 0.46). There was a tendency for OW to display a greater total fat oxidation than IBAN in PH, although this did not reach statistical significance (10.6 § 1.54 g versus 9.0
- 3.1 g; ES 0.7; p D 0.13).
Continuous glucose monitoring
Owing to instances of poor compliance to diet replica tion and sensor malfunction and drop out, CGM anal ysis was only completed on eight participants, with an unequal split between IBAN (n D 3) and OW (n D 5). Therefore, it was only possible to conduct analysis on PH compared to EX Peak glucose concen tration in response to the meal following PH (6.3 §1.4 mmol¢:.} 1) was lower compared to the same meal after EX (6.8 § 1.2 mmol9t) 1; p < 0.05, CID j 0.9 to 1.9, ES D 0.4, Figure 3A). There was no difference in glucose AUC for PH compared to EX following this meal (p D 0.875, CI D i 11.2 to 12.4, ES D 0.1 Figure 3B). There were no differences in either peak glucose (meal 2 p D 0.168, meal 3 p D 0.266) or AUC (meal 2 p D 0.070, meal 3 p D 0.183) consumed in the subsequent 24-hour period. There were also no differ ences in total 24-hour AUC between PH and EX (p D 0.168)
Core temperature
There was a main effect of time (p < 0.0001) and an interaction effect (P < 0.0001), but no main effect of condition on DTc (p D 0.112, Figure 4a). PH DTc was lower in OW compared to IBAN from 35 minutes (p < 0.05), although by 60 minutes there was no dif ference in DTc (1.0 § o.2Ue versus 0.9 § 0.2 , LEAN versus OW, respectively p D 0.089, CI D j 0.13 to 0.33, ES D 0.5). There were no differences in DTc between OW and IBAN during EX at any time point, with DTc at 60 minutes being 0.8 § 0.1Lle versus 0.8 §o.2Ue for LEAN and OW, respectively (p D 0.999). S<in temperature There were main effects of time (p < 0.0001), condi tion (p < 0.0001) and an interaction effect (p < 0.0001) on DT s< (Figure 4B). There were no differen ces in DTs< between OW and LEAN following EX (LEAN 1.7 § 0.7U@; versus OW 1.7 § l.6Ue;, p D 0.999, ES D 0.0) or PH (LEAN 4.2 § o.4Ue; versus OW 4.7 § 0.6 , pD 0.095, ES D 0.9). Muscle temperature There was an effect of depth on DTm (p < 0.001), but no effect of condition (p D 0.285) or interaction (p D 0.111, Figure 4C). Following PH, the DTm was greater for LEAN compared to OW at a depth oflcm (LEAN, 2.5 § 0.8Ue; OW 1.9 § 0.6Lie;, p < 0.0005, CI D i 0.2 to 1.4, ES D 0.8) and 3cm (LEAN, 2.3 § 0.6U@;; OW 1.9 § 0.7, p < 0.005, CID i 0.4 to 1.2, ES D 0.6). After EX, DTm was only different at 1cm (LEAN, 2.3 § 0.6Ue;; OW 2.0 § 0.7, p < 0.005, CI D i 0.5 to 1.1, ES D 0.4).
Mean body temperature
There was a difference in DTb between conditions (p < 0.0001, Figure 4D). There were differences between LEAN PH and LEAN EX (1.7 § 0.3iJe: versus 1.1 § 0.3iJe:, p < 0.005, CI D 0.3 to 1.0, ES D 1.9); OW PH and OW EX (1.8 § o.21J@: versus 1.0 § 0.4Lle; p < 0.001, CI D 0.4 to 1.2, ES D 2.4). There were no differences between LEAN and OW within the same condition (both p > 0.9).
Heart rate
There were significant effects of time, condition and interaction effect of heart rate (all p < 0.0001). Mean percentage HRmax EX was 78 § 8% compared to 54 § 7% in PH (p < 0.0001). There were no differences in the mean percentage of HRmax between groups for either PH (LEAN D 54.0 § 6.0% versus OW D 55.2
- 6.2%, p D 0.719) or EX (LEAN D 78.5 § 7.3%; OW
D 81.7 § 7.4%, p D 0.428).
Discussion
The primary outcome of the present study was that the concentration of eHSP70 in the plasma was similar between EX and PH when both conditions were matched for DTc, with DTm being the best individual predictor of DeHSP7O, explaining » 25% of the total variance. This suggests that changes to Tm may in part determine the appearance of eHSP7O. An important secondary outcome is the increase in energy expendi ture evident as a consequence of PH. This has impor tant implications for the use of PH as a future intervention that may help to moderate body mass, particularly for individuals unable or unwilling to complete regular physical activity. However, we recog nize that PH should be coupled with dietary manipu lation to maximize any potential benefit to body composition. Finally, the reduction in peak glucose in the meal following PH compared to EX suggests that PH may have a beneficial effect on glycemic regulation and provide an alternative or augmentative interven tion by which individuals with metabolic diseases may be able to improve their glycemic control. However, additional research needs to be conducted to confirm these data in larger cohorts over a prolonged period of time.
The HSP7O response may be hindered by excess body mass. Regression analysis demonstrated a link between body mass and the DeHSP7O. A greater body mass was associated with an impaired eHSP7O response to heating, via either EX or PH, explaining 16% of the total variance. Such variation may be indic ative of an impaired HSP response in overweight individuals in the face of physiological stress and would likely be compounded by low aerobic capacity.46 The implications for such a deficiency are wide ranging and may increase susceptibility to metabolic disease,46,47 impaired vascular function48 and even contribute to the impaired thermoregulatory control evident in individuals with diabetes.49 While there were no clear differences in the HSP7O response between either PH or EX, there were clear differences in the IL-6 response. Previous work has shown that temperature increases independently ele vate IL-6.50However, the present data do not support the view that skeletal muscle may act as a “heat stress sensor” triggering a subsequent cytokine response, as none of the thermal measures taken during the trials were effective predictors of DIL-6 and could not account for a significant proportion of the variation in IL-6 following heating. Our data also indicate that the difference in IL-6 between PH and EX is not due to HSP7O induction of IL-6,35’36 as DHSP7O was similar following PH and EX This suggests the IL-6 response to EX occurs independently ofHSP7O expression and is more dependent on intensity and duration of EX,51 than exposure to heat stress. IL-6 combined with its receptor (IL-6R), may also have a positive effect on insulin independent glucose uptake at rest.52 Should chronic PH enhance IL-6R expression as is evident following EX training, 53 interventions capable of increasing both IL-6 and IL-6R, may provide a thera peutic basis for improving glycemic control. Moreover, it has recently been shown that acute PH elevates the anti-inflammatory cytokines IL-lra and IL-10,54 suggesting that chronic PH may benefit the inflammatory profile in a similar way to EX However, long-term investigation of PH is required to corrobo rate this hypothesis.
Although the sample size for analysis of the glyce mic response to PH and EX was somewhat limited, the fact that comparable glycemic profiles were evi dent between PH and EX is interesting. Given the acute effect of EX on improving glucose control as a consequence of improved insulin sensitivity5,5this is a somewhat surprising result. However, it has recently been shown that heat stress activates insulin indepen dent glucose transport via s0 adenosine monophos phate-activated protein kinase (AMPK) activation. 56 In response to the heat stress stimulus, a reduction in muscle adenosine triphosphate, phosphocreatine and glycogen was reported, indicating a significant energy cost to the heating protocol and hence activation of AMPK This suggests that, acutely at least, heat stress may improve glucose uptake due to increased energy expenditure. Further work needs to be conducted to establish the exact mechanisms by which PH may exert such an effect. In addition to benefits to metabolic regulation, PH has been shown to benefit cardiovascular health. In a recent meta-analysis, Laukkanen et al. report that life long sauna use reduced cardiovascular and all cause mortality, with the largest benefits associated with more frequent sauna use.57 Although this relationship does not establish causality,31’58 it is nonetheless an interesting link that is supported by recent investiga tions.59-61 PH appears to improve vascular : function, with improvements to flow-mediated dilatation, arterial stiffuess and blood pressure reported after 8 weeks of PH.59 Moreover, Tho as et al. have shown that 30 ;minutes of PH can induce a significant shear stress response and a reduction in mean arterial pressure in patients with peripheral arterial disease.62 Further more, in a healthy cohort who underwent matched duration PH or EX, the PH group demonstrated a greater shear stress response compared to that in response to EX60 Based on heart rate responses, the cardiovascular strain in the present study was compa rable to that of Thomas et al., which elicited » 50% HRmax during their immersion protocol.60 These recent findings indicate that PH has the potential to induce significant systemic benefits, which in some instances may be comparable to or even greater than the effects evident following EX. There are a few limitations with the present investi gation. First, the omission of a measure of iHSP7O, either derived from skeletal muscle or leukocytes malces it difficult to draw firm conclusions on the potential intracellular effect of PH and glycemic regu lation. Without direct measures ofiHSP7O, in addition to JNK and other molecules in the insulin-signaling cascade, it is difficult to derive the exact effect of PH on acute glycemic control. Therefore, we are limited to discuss the relationship between eHSP7O and iHSP7O and that an increase in the extracellular com ponent is reflective of changes in the intracellular compartment. Future investigation should employ techniques to measure both iHSP7O and eHSP7O to enable greater understanding of the effect of PH on the intracellular to extracellular ratio. Second, the rela tively small sample size of this study, which may limit the statistical power of our experiments. We acknowl edge that further experiments with larger cohorts should be conducted in order to replicate the present data.
In summary, the data presented here demonstrate that both acute PH and EX result in comparable increases in HSP7O. It is suggested that DHSP7O is sensitive to the DTm· The elevations in both HSP7O and IL-6 may promote an anti-inflammatory milieu and help combat the chronic inflammation associated with many disease states. The increase in energy expenditure following PH demonstrates a systemic effect of PH, which has the ability to reduce adipose tissue and further contribute to reducing inflammation. However, it should be noted that EX still offers the greatest overall benefit to weight control and metabolic health. Finally, in agreement with that of other authors, the present data indicate that PH might provide an alternative to EX in some individuals who are too physically impaired to undertake prolonged aerobic activity to improve their cardio-metabolic health. The possible therapeutic benefits of PH require more extensive investigation.
Abbreviations
AM:PK s0 adenosine monophosphate-activated protein kinase Area under the curve Body mass index blood pressure body surface area Continuous glucose monitor confidence interval coefficient of variation extracellular heat shock protein enzyme-linked immunosorbent sandwich assay effect size Exercise trial High-density lipoprotein cholesterol Metabolic heat production Heat shock protein Intracellular heat shock protein Interleukin 1 receptor antagonist Interleukin 6 Interleukin 10 c-Jun N-terminal kinase Low density lipoprotein cholesterol Lean participant group Overweight participant group Passive heating trial Standard deviation Standard error of mean Type 2 diabetes mellitus body temperature core temperature muscle temperature skin temperature Triglyceride carbon dioxide production oxygen uptake Maximum volume of oxygen uptake change in temperature Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank; Prof George Havenith for granting access to the Environmental Ergonomics Research Centre and Dr Caroline Smith for her insightful discussion and comments during the preparation of the manuscript.
Funding
The research was also partly supported by the National Insti tute for Health Research (NIHR) Diet, Lifestyle & Physical Activity Biomedical Research Unit based at University Hospitals of Leicester and Loughborough University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
ORCID
- H. Faulkner http://orcid.org/0000-0003-4688-7252 C. A. Leicht http:l/orcid.org/0000-0002-3539-8480
References
- Martin LR, Williams SL, Haskard KB, DiMatteo MR The challenge of patient adherence. Ther Clin Risk Man. 2005;1(3):189-199.
- Praet SFE, van Rooij E.S.J., Wijtvliet A, Boonman-de Winter LJM, Enneking Th, Kuipers H, Stehouwer CDA, van Loon Brisk walking compared with an individualised medical fitness programme for patients with type 2 diabetes: a randomized controlled trial. Diabetologia. 2008;51(5):736-746. PMID:18297259; doi:10.1007/s00125- 008-0950-y.
- Jill A comparison of views of individuals with type 2 diabetes me!litus and diabetes educators about barriers to diet and exercise. J Health Commun. 2001;6(2):99-115. PMID:11405082; doi:10.1080/10810730116985.
- Febbraio MA, Mesa JL, Chung J, Steensberg A, Keller C, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein 72 and heat shock protein 60 in humans. Cell Stress Chaperones. 2004;9(4):390-396. PMID:15633297; doi:10.1379/CSC- 24Rl.1.
- McCarty Induction of heat shock proteins may combat insulin resistance. Med Hypotheses. 2006;66(3):527-534. PMID:16309849; doi:10.1016/j.mehy.2004.08.033.[ 6] McCarty MF, Barroso-Aranda J, Contreras F.Regular ther mal therapy may promote insulin sensitivity while boosting expression of endothelial nitric oxide synthase – effects comparable to those of exercise training. Med Hypotheses. 2009; 73(1):103-105. PMID:19203842; doi:10.1016/j.mehy. 2008.12.020.
- KrauseM,LudwigMS,HeckTG,TakahashiHKHeatshock proteins and heat therapy for type 2 diabetes: pros and Curr Opin Clin Nutr Metab Care. 2015;18(4):374-380. PMID:26049635; doi:10.1097/MCO.0000000000000183.
- Krause M, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, de Bittencourt PIH. The chaper one balance hypothesis: the importance of the extracellu lar to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediators Inflamm. 2015;2015:249205. doi:10.1155/2015/249205.
- Rodrigues-Krause J, Krause M, O’Hagan C, De Vito G, Boreham C, Murphy C, Newsholme P, Colleran G. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17(3):293-302. PMID :22215518; doi:10.1007I s12192-0ll-0319-x.
- Molina MN, Ferder L, Manucha Emerging role of nitric oxide and heat shock proteins in insulin resisance. Curr Hypertens Rep. 2016;18(1):1-015-0615-4. doi:10.1007/sl 1906-015-0615-4.
- Ortega E, Bote ME, Besedovsky HO, Rey Ad. Hsp72, inflammation, and aging: causes, consequences, and perspectives. Ann NY Acad 2012;1261(1):64-71. PMID: 22823395; doi:10.1l ll/j.1749-6632.2012.06619.x.
- Yamada P, Amorim F, Moseley P, Schneider S. Heat shock protein 72 response to exercise in humans. Sports 2008;38(9):715-733. PMID:18712940; doi:10.2165/ 00007256-200838090-00002.
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Har greaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6 (4):386-393. PMID:11795476; doi:10.1379/1466-1268 (2001)006%3c0386:EISHIH%3e2.0.CO;2.
- Riezman H. Why do cells require heat shock proteins to survive heat stress? Cell Cycle. 2004;3(1):60-62. doi:10.4161/cc.3.1.625
- Hooper PL, Hooper PL. Inflammation, heat shock pro teins, and type 2 diabetes. Cell Stress Chaperones. 2009; 14(2):113-115. PMID:18720028; doi:10.1007/s12192- 008-0073-x.
- Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S. Heat shock proteins in diabetes and wound healing. Curr Protein Pept 2009;10(1):85-95. PMID: 19275675; doi:10.2174/138920309787315202.
- Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol. 2011; 110(2):451-457. PMID:21148343; doi:10.1152/ japplphysiol.00849.2010.
- Chung J, Nguyen A, Henstridge DC, Holmes AG, Stanley Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, et al. HSP72 protects against obesity induced insulin resistance. PNAS. 2008;105(5):1739- PMID:18223156; doi:10.1073/pnas.0705799105.
- Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002; 51(4):1102-1109. doi:10.2337/diabetes.51.4.l102
- Periard JD, Ruell P, Caillaud C, Thompson Plasma Hsp72 (HSPAlA) and Hsp27 (HSPBl) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones. 2012;17(3):375-383. PMID:22222935; doi:10.1007/sl2192-0l l-0313-3.
- Fehrenbach E, Niess AM, Veith R, Dickhuth HH, North ruce CR, Carey AL, Hawley JA, Febbraio MA. Intra muscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003; 52(9):2338-2345. PMID:12941774; doi:10.2337/diabetes.52.9.2338. off H. Changes of HSP72-expression in leukocytes are associated with adaptation to exercise under conditions of high environmental temperature. J Leukoc Biol. 2001; 69(5):747-754. PMID:11358983.
- Gibson 0, Dennis A, Parfitt T, Taylor L, Watt P, Maxwell
- Extracellular Hsp72 concentration relates to a minimum endogenous criteria during acute exercise-heat exposure. Cell Stress and Chaperones. 2014;19(3):389- 400. PlvfID:24085588; doi:10.1007/sl2192-0l3-0468-l.
- Iguchi M, Littmann AB, Chang SH, Wester LA, Knipper
- JS, Shields RK. Heat stress and cardiovascular, hormonal, and heat shock proteins in humans. J Athl Train. 2012; 47(2):184-190. PMID:22488284.
- Bathaie SZ, Jafurnejad A, Hosseinkhani S, Nakhjavani M. The efiect of hot-tub therapy on serum Hsp70 level and its benefit on diabetic rats: a preliminary Int JHyperther. 2010;26(6):577-585. doi:10.3109/02656736.2010.485594.
- Karpe PA, Tikoo K. Heat shock prevents insulin Resistance-Induced vascular complications by augmenting angiotensin-(1-7) signaling. Diabetes. 2014;63(3):1124- doi:10.2337/dbl3-1267.
- Gupte AA, BomhoffGL, Geiger Age-related differences in skeletal muscle insulin signaling: the role of stress kinases and heat shock proteins. J Appl Physiol (1985). 2008;105(3) :839-848. doi:10.l 152/japplphysiol.00148. 2008.
- Raz I, Weiss R, Yegorchikov Y, Bitton G, Nagar R, Pesach
- Effect of a local heating device on insulin and glucose pharmacokinetic profiles in an open-label, randomized, two-period, one-way crossover study in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Clin Ther. 2009;31(5):980-987. PMID:19539098; doi:10.1016/j.clinthera2009.05.010.
- Hermanns N, Bitton G, Reimer A, Krichbaum M, Kulzer B, Haak Effect of local heating on postprandial blood glucose excursions using the InsuPad device: results of an outpatient crossover study J Diabetes Sci Technol. 2014; 8(6):1126-1132. PMID:25113814; doi:10.1177/ 1932296814546162.
- Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341(12):924-925. PMID:10498473; doi:10.1056/NEJM199909163411216.
- Welc SS, Phillips NA, Oca-Cossio J, Wallet SM, Chen DL, Clanton TL. Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am J Physiol Cell Physiol. 2012; 303(4):C455-C466. PMID:22673618; doi:10.1152/ ajpcell.00028.2012.
- Helge J, Stallknecht B, Pedersen BK,.Galbo H, Kiens B, Richter The effect of graded exercise on II.r6 release and glucose uptake in human skeletal muscle. J Physiol (Lond). 2002;546:299-305. doi:10.1113/jphysiol.2002. 030437.
- Saini A, Faulkner SH, Moir H, Warwick P, King J, Nimmo MA. Interleukin-6 in combination with the interleukin-6 receptor stimulates glucose uptake in rest ing human skeletal muscle independently of insulin action. Diabetes, Obes Metab. 2014;16(10):931-936. doi:10.1111/dom.12299.
- Keller C, Steensberg A, Hansen AK, Fischer CP, Plom gaard P, Pedersen BK Effect of exercise, training, and gly cogen availability on II.r6 receptor expression in human Jay 0, Gariepy IM, Reardon FD, Webb P, Ducharme MB,·Ramsay T, Kenny GP. A three-compartment ther mometry model for the improved estimation of changes in body heat content. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R167-175. PMID:16931653; doi:10.1152/ajpregu.00338.2006.
- Batterham AM, Hopkins WG. Making meaningful inferences about magnitudes. Int J Sports Physiol Per form. 2006;1(1):50-57. PMID:19114737; doi:10.1123/ ijspp.1.1.50.
- Rogers RS, Morris EM, Wheatley JL, Archer AE, McCoins CS, White KS, Wilson DR, Meers GM, Koch LG, Britton SL, et al. Deficiency in the heat stress response could underlie susceptibility to metabolic dis ease. Diabetes. 2016. doi:10.2337/db16-0292.
- Kondo T, Motoshima H, Igata M, Kawashima J, Matsu mura T, Kai H, Araki E. The role of heat shock response in insulin resistance and diabetes. Diabetes Metab 2014; 38(2):100-106. PMID:24851203; doi:10.4093/ dmj.2014.38.2.100.
- Malyshev IY, Bayda LA, Trifonov AI, Larionov NP, Kubrina LD, Mikoyan VD, Vanin AF, Manukhina EB. Cross-talk between nitric oxide and HSP70 in the antihy potensive effect of adaptation to heat. Physiol Res. 2000; 49(1):99-105. PMID:10805410.
- Kenny GP, Sigal RJ, McGinn Body temperature regulation in diabetes. Temperature. 2016;3(1):119-145. doi:10.1080/23328940.2016.1230171.skeletal muscle. J Appl Physiol. 2005;99(6):2075-2079. PMID:16099893; doi:10.1152/japplphysiol.00590.2005.
- Leicht CA, Papanagopoulos A, Haghighat S, Faullmer SH. Increasing heat storage by wearing extra clothing during upper body exercise up-regulates heat shock protein 70 but does not modify the cytokine response. J Sports 2016;1-7; doi:10.1080/026404l 4.2016.1235795.
- Goodyear LJ, Kahn BB. Exercise, glucose transport and insulin sensitivity. Annu Rev Med. 1998;49(1):235-261. PMID:9509261; doi:10.1146/annurev.med.49.1.235.
- Goto A, Egawa T, Sakon I, Oshima R, Ito K, Serizawa Y, Sekine K, Tsuda S, Goto K, Hayashi T. Heat stress acutely activates insu!inrnih.dependent glucose transport and 5<rnlMPOOctivated protein kinase prior to an increase in HSP72 protein in rat skeletal muscle. Physiol Rep. 2015;3(11). doi:10.14814/phy2.12601.
- Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med. 2015; 175(4):542-548. PMID:25705824; doi:10.1001/jamainternmed.2014.8187.
- Kivimaki M, Virtanen M, Ferrie The link between sauna bathing and mortality may be noncausal. JAMA Internal Medicine. 2015;175(10):1718-1718. PMID: 26436738; doi:10.1001/jamainternmed.2015.3426.
- Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary J Physiol (Land). 2016;594(18):5329-5342. PMID: 27270841; doi:10.l113/JP272453.
- Thomas KN, van Rij AM, Lucas SIB, Gray AR, Cotter Substantive hemodynamic and thermal strain upon completing lower-limb hot-water immersion; compari sons with treadmill running. Temperature. 2016; 3(2):286-297. doi:10.1080/23328940.2016.1156215.
- Romero SA, Gagnon D, Adams AN, Cramer MN, Kouda K, Crandall CG. Acute limb heating improves macro and microvascular dilator function in the leg of aged humans. Am J Physiol Heart Circ Physiol. 2017;312(1): H89-H97. PMID:27836894; doi:10.1152/ajpheart. 00519.2016.
- Thomas KN, van Rij AM, Lucas SIB, Cotter Lower limb hot-water immersion acutely induces beneficial hemodynamic and cardiovascular responses in peripheral arterial disease and healthy, elderly controls. Am J Phys iol Regul Integr Comp Physiol. 2017;312(3):R281-R291. doi:10.1152/ajpregu.00404.2016.
