Andrew P. Hunt1,2*, Geoffrey M. Minett1,2, Oliver R. Gibson3,4, Graham K. Kerr1,2 and Ian B. Stewart1,2
1 School of Exercise and Nutrition Sciences, Faculty of Health, Queensland University of Technology, Brisbane, QLD, Australia, 2 Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, QLD, Australia, 3 Centre for Human Performance, Exercise and Rehabilitation, College of Health and Life Sciences, Brunel University
London, Uxbridge, United Kingdom, 4 Division of Sport, Health and Exercise Sciences, Department of Life Sciences, College of Health and Life Sciences, Brunel University London, Uxbridge, United Kingdom
Neurodegenerative diseases involve the progressive deterioration of structures within the central nervous system responsible for motor control, cognition, and autonomic function. Alzheimer’s disease and Parkinson’s disease are among the most common neurodegenerative disease and have an increasing prevalence over the age of 50. Central in the pathophysiology of these neurodegenerative diseases is the loss of protein homeostasis, resulting in misfolding and aggregation of damaged proteins. An element of the protein homeostasis network that prevents the dysregulation associated
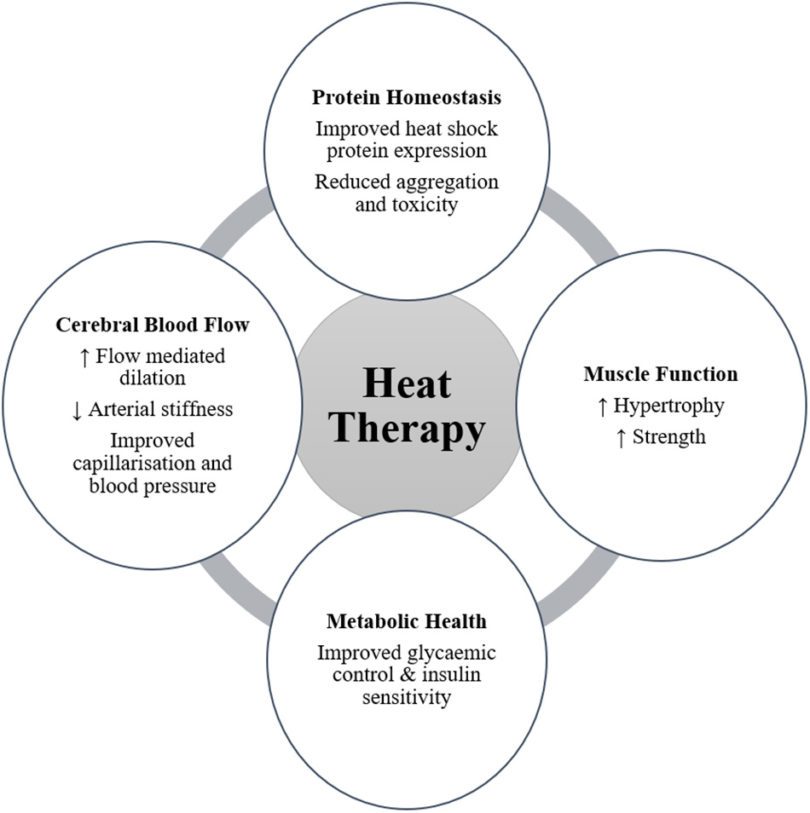
with neurodegeneration is the role of molecular chaperones. Heat shock proteins (HSPs) are chaperones that regulate the aggregation and disaggregation of proteins in intracellular and extracellular spaces, and evidence supports their protective effect against protein aggregation common to neurodegenerative diseases. Consequently, upregulation of HSPs, such as HSP70, may be a target for therapeutic intervention for protection against neurodegeneration. A novel therapeutic intervention to increase the expression of HSP may be found in heat therapy and/or heat acclimation. In healthy populations, these interventions have been shown to increase HSP expression. Elevated HSP may have central therapeutic effects, preventing or reducing the toxicity of protein aggregation, and/or peripherally by enhancing neuromuscular function. Broader physiological responses to heat therapy have also been identified and include improvements in muscle function, cerebral blood flow, and markers of metabolic health. These outcomes may also have a significant benefit for people with neurodegenerative disease. While there is limited research into body warming in patient populations, regular passive heating (sauna bathing) has been associated with a reduced risk of developing neurodegenerative disease. Therefore, the emerging evidence is compelling and warrants further investigation of the potential benefits of heat acclimation and passive heat therapy for sufferers of neurodegenerative diseases.
INTRODUCTION
Humans are homeothermic and as such regulate their core body temperature within a narrow range. Perturbations to this homeostasis, induced by external environmental thermal stress or internally generated metabolic heat, produces both autonomic and behavioral responses designed to elicit a return of core body temperature toward thermal balance (Schlader and Vargas, 2019). While in an acute sense this stress response is a defense mechanism, regularly challenging the thermal equilibrium via active or passive thermal stress results in positive physiological and perceptual adaptations (Tyler et al., 2016). Recent research has shown positive therapeutic effects of passive heating for
people with peripheral arterial disease (Neff et al., 2016; Akerman et al., 2019), chronic heart failure (Kihara et al., 2002; Ohori et al., 2012), diabetes (Hooper, 1999), and depression (Janssen et al., 2016). Passive heating also improves a range of health markers, including cardiovascular health indices, such as vascular function, blood pressure, and arterial stiffness (Brunt et al., 2016a,b), as well as metabolic health and glycemic control
(Janssen et al., 2016; Kimball et al., 2018; Ely et al., 2019; Maley et al., 2019). Several mechanistic pathways may underpin these adaptations, including improved cellular respiration (Hafen et al., 2018), circulating factors (Brunt et al., 2019), and vascular shear stress (Tinken et al., 2009; Thomas et al., 2016). The upregulation of heat shock proteins (HSPs) as a result of acute and/or chronic (repeated) exposure to passive heating is also an adaptive
outcome, which may provide a specific mechanistic pathway for improving health and function within the body (Faulkner et al., 2017; Brunt et al., 2018). Recent reviews have identified the upregulation of HSPs as therapeutic targets for the treatment of neurodegenerative diseases including Parkinson’s disease and Alzheimer’s disease (Carman et al., 2013; Kalmar et al., 2014; Schapira et al., 2014; Ciechanover and Kwon, 2017; Webster et al., 2017; Klaips et al., 2018). Neurodegenerative diseases are characterized by the progressive deterioration of structures within the central nervous system responsible for motor control, cognition, and
autonomic function. Alzheimer’s and Parkinson’s diseases are among the most common neurodegenerative diseases and have an increasing prevalence over the age of 50 (Pringsheim et al., 2014). Loss of protein homeostasis, due to protein mis-folding and aggregation of damaged proteins, is a hallmark of both Alzheimer’s and Parkinson’s diseases (Labbadia and Morimoto, 2015). HSPs function as chaperones to ensure appropriate cell function with distinct roles in the unfolded protein response, recognizing misfolded or mis-localized proteins that may be
subsequently degraded by the proteasome, and are a key component of chaperone-mediated autophagy (Adachi et al., 2009; Stetler et al., 2010; Leak, 2014; Zarouchlioti et al., 2018). For their role in regulating protein homeostasis, HSP expression has been proposed as a therapeutic target for the treatment of these neurodegenerative diseases (Carman et al., 2013; Kalmar et al., 2014; Schapira et al., 2014; Ciechanover and Kwon, 2017; Webster et al., 2017; Klaips et al., 2018). As physical and cognitive ability decline in Alzheimer’s and Parkinson’s diseases, passive heat therapy may yield an achievable alternative to the presently recommended exercise interventions in this population. Intriguingly, the incidence of Alzheimer’s disease has recently been shown to be reduced in people who undertook moderate to frequent sauna bathing (Laukkanen et al., 2017). While the current evidence for heat therapy in neurodegenerative disease is associative and the mechanisms by which improved health outcomes are achieved have yet to be elucidated, the potential of passive heating in this population remains an alluring therapeutic option. This review will examine pathophysiological determinants of common neurodegenerative disease, examine the evidence of an elevated HSP expression as a potential therapeutic intervention in common neurodegenerative diseases, and describe the role heat acclimation and passive heat therapy have in inducing HSP expression. In addition, central and peripheral adaptations to
body warming in healthy adults, including improved muscular function, cerebral blood flow, and metabolic health, will be considered with their potential influence on neurodegenerative disease outcomes. Finally, considerations for undertaking heat acclimation and/or passive heating interventions in people with neurodegenerative diseases will be addressed.
CLICK HERE to continue reading paper